Our Pipeline Is Designed To Address Critical Unmet Needs
Our Highlights to Date
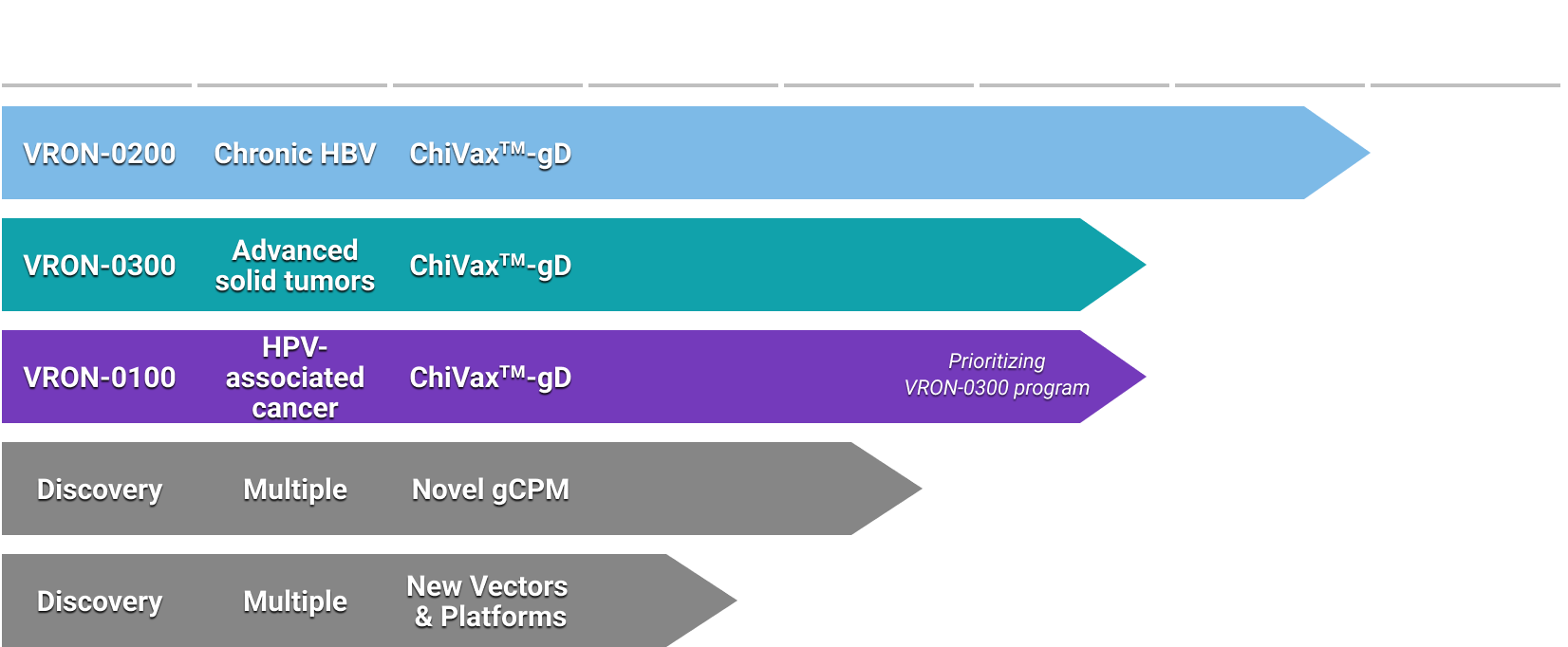
Clinical Development Program
- VRON-0200: HBV clinical stage program
- Phase 1b international clinical study now enrolling (NCT06070051)
- drug products manufactured and available
- country regulatory submissions: 2 – approved, 1 pending
- study sites: 2 – US; New Zealand and Hong Kong active and enrolling
- VRON-0300: Oncology program
- IND-enabling studies underway
- Extensive preclinical oncology data (presented at ESMO TAT 2023; accepted for SITC 2023)
- Translational and clinical development plans and potential collaborations are progressing
- Other
- Top 5% of all abstracts at EASL Congress 2023 (HBV)
- Dr. Ertl – Rosalind Franklin Society Award Winner
- Pennsylvania State Innovation Zone Awardee
- Strategic collaboration with Center for Breakthrough Medicine (CDMO)
VRON-0200: Treatment of HBV Infection
The T Cell Platform for Multi-Modal Therapies & Functional Cure
VRON-0200 is designed to address a global high unmet medical need for chronic HBV infection. VRON-0200 combines a genetically encoded checkpoint modifier along with selected and optimized antigens of choice and delivered by ChiVax™, our heterologous chimpanzee adenoviral vectors that promotes potent, prolonged, and broad CD8+ T cell responses.
Platform Technologies
- Preclinical studies with the lead checkpoint modifier: HBV, HPV, melanoma, influenza virus, HIV and COVID-191,3–7
- Clinical studies: NIH sponsored Phase 1 HIV Prevention trial (South Africa) using our vectors8
- Future global health and pandemic needs: The ChiVax™ platform can quickly respond and adapt to help address current and future global public health pandemic and other infectious diseases needs
CD, cluster of differentiation; FDA, Food and Drug Administration; gCPM, genetically encoded checkpoint modifier; gD, glycoprotein D; HBV, hepatitis B virus; HIV, human immunodeficiency virus; HPV, human papillomavirus; IND, investigational new drug; MOA, mechanism of action; NIH, National Institutes of Health.
- Hasanpourghadi M, et al. EASL 2021:Abstract OS-2478.
- Hasanpourghadi M, et al. APASL 2022:Abstract OS-0685.
- Xiang Z, et al. ASCO-SITC Clinical Immuno-Oncology Symposium 2020:Abstract 71.
- Zhang Y and Ertl HCJ. J Immunol. 2014;193:1836–46.
- Dawany N, et al. Aging (Albany NY). 2016;8:3727–97.
- Tuyishime S, et al. EBioMedicine. 2018;31:25–35.
- Novikov M, et al. BioRxiv. Published online January 1, 2022. DOI:10.1101/2022.02.23.481620.
- HIV Vaccine Trials Network (HVTN)-139 trial, South African Medical Research Council.