Our Innovative Approach to T Cell-Based Immunotherapies
Driven by Science, We Created a Unique Iterative Process to Designing Disease-Specific Immunotherapies

Step 1. Have we identified the right target?
First, we conduct extensive research on the target disease and the indication to ensure the end product addresses a high unmet clinical need, is technologically feasible, and is differentiated from other available therapies, including those in development.

Step 2. What are the optimal components?
Monoclonal antibodies target the rescue of activated, but exhausted, CD8+ T cells.1 Immunotherapies that can alter (and optimize) CD8+ T cell activation have the potential to enhance and broaden T cell responses.1–3 We intelligently select and optimize the antigens of choice. By this, we mean that we conduct extensive literature reviews and research, followed by rigorous preclinical studies to identify the right antigen to maximize its potential therapeutic benefit, before we combine them with a genetically encoded checkpoint modifier that works to boost T cell activation. Both components are delivered via ChiVax™, our adaptable heterologous chimpanzee adenoviral vectors.

Step 3. Are we providing the best therapeutic potential?
Through rigorous testing and analysis, each proprietary adenoviral construct is optimized to provide the best possible therapeutic potential for its specific target indication.

Step 4. Does the therapeutic potential translate into clinical benefit?
Our novel checkpoint modifiers are designed with the goal of maximizing benefits to patients. To achieve this, we ensure that our clinical trial programs are designed to consider the registrational path of the therapy, that we refine and target the specific patient population, and confirm it all through a proof of concept human clinical trial read out. Through intelligent and science-driven processes, we give our T-cell based immunotherapies the best chance to succeed and achieve our vision of transforming the treatment landscape for patients with cancer and chronic infectious diseases.
It starts with the science
An interview with Dr. Hildegund Ertl, Scientific Founder of Virion Therapeutics, LLC, and a Professor in the Vaccine & Immunotherapy Center at The Wistar Institute, about her research interests in immunology and vaccines and her achievements towards the scientific advancement for treating cancer and infectious disease.
Our Platform: VIACT™
Virion’s Intelligent and Adaptable CD8+ T cell-based Immunotherapy (VIACT™) platform combines genetically encoded checkpoint modifiers, with intelligently selected and optimized target antigens, inserted into heterologous chimpanzee adenoviral vectors – inducing potent, prolonged, broad, and highly functional CD8+ T cell responses.
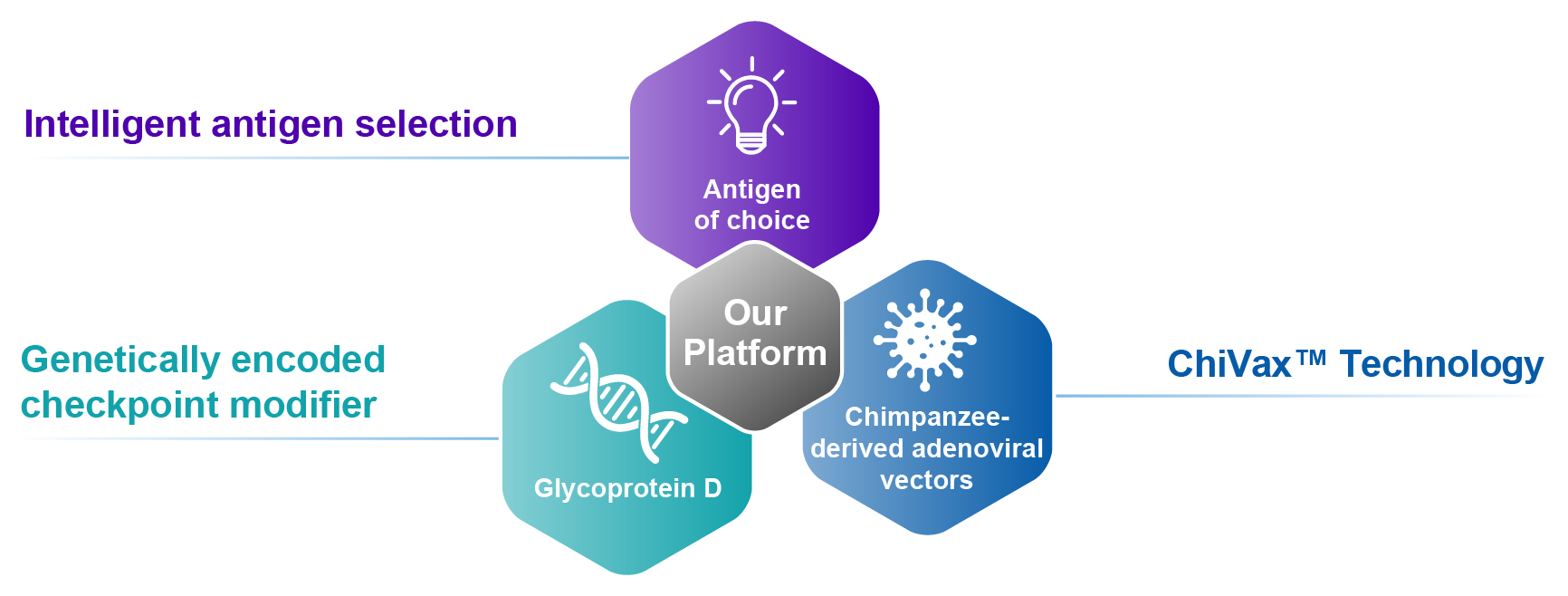
Intelligent Antigen Selection
CD8+ T cell responses are critical for an immune response to cancer and infectious disease. Our platform has selected and optimized target-specific antigens across various cancers and infectious diseases.
Checkpoint Modifiers
Our genetically encoded checkpoint modifiers can enhance CD8+ T cell responses to target disease-specific antigens. Preclinical animal models show that our checkpoint modifiers lower the activation threshold of T cells upon vaccination. This produces potent, broad, and durable CD8+ T cell responses.

ChiVax™ Technology
Heterologous chimpanzee adenoviral vectors were pioneered by members of the Virion Therapeutics team. Heterologous adenoviral vectors are thought to enhance and broaden T cell responses, particularly in combination with our checkpoint modifiers. They are now in clinical use to combat multiple human diseases, including COVID-19.
Presentations & Publications
More information about our technologies and research is available in our publications and scientific presentations library.
CD, cluster of differentiation.
- Lee J, et al. For Immunopathol Dis Therap. 2015;6(1-2):7–17.
- Xiang ZQ, et al. ASCO-SITC Clinical Immuno-Oncology Symposium 2020:Abstract 71.
- Virion Therapeutics. Data on file.