T cell-based Immunotherapies
for Cancer and Infectious Diseases
Our Company
Virion is a clinical-stage biopharmaceutical company
Virion Therapeutics is a clinical-stage company developing novel T cell-based immunotherapies for cancer and chronic infectious diseases. We utilize genetically encoded checkpoint modifiers (CPM) to enhance and broaden CD8+ T cell responses to a tumor or chronic infection. In preclinical models of different diseases, addition of CPMs resulted in improved clinical outcomes and survival. Human studies using the first CPM in subjects with chronic HBV are now enrolling (Q3 2023).
Our Science
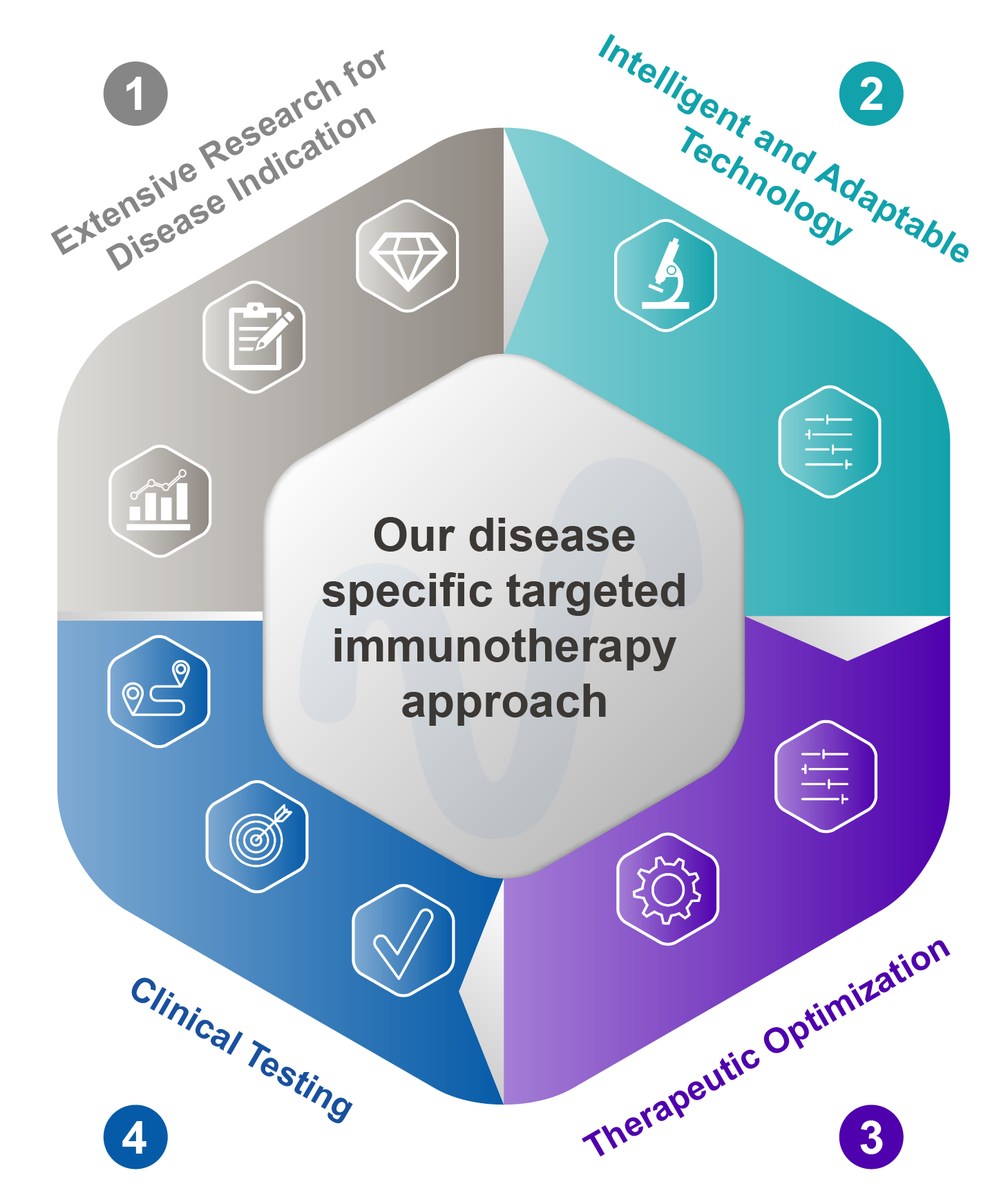
A New Therapeutic Approach to Treating Cancer and Infectious Diseases
The discovery and use of checkpoint inhibitors has revolutionized cancer treatment. But immunotherapies that can optimize CD8+ T cell responses have the potential to further improve clinical outcomes for many infectious diseases and cancers. Our iterative scientific approach is designed to produce novel, adaptable, and accessible disease-specific CD8+ T cell-based immunotherapies.
Our Pipeline
Virion Has a Series of Programs in Development
A first-in-human study for our lead VRON-0200 HBV program is now open for enrollment (Q3 2023). This and future clinical studies will evaluate our therapeutic approach using our novel VIACT™ platform, which combines genetically encoded checkpoint modifiers with intelligently selected and optimized target antigens, inserted into heterologous chimpanzee adenoviral vectors.